


Results &
Side Effects
Actor portrayal
Our clinical trial was designed with the HAE community in mind
The KONFIDENT trial included 110 patients with type I or type II HAE who treated their attacks with either EKTERLY or placebo.
97 adults and 13 CHILDREN
(12 YEARS OF
AGE
AND OLDER)
-
23% of people
treated with both preventative and
on-demand treatments -

77% of people
treated with
on-demand treatment only
People in the study had at least 2 or more attacks
in the 3
months leading up to the trial, including those
on preventative treatment.
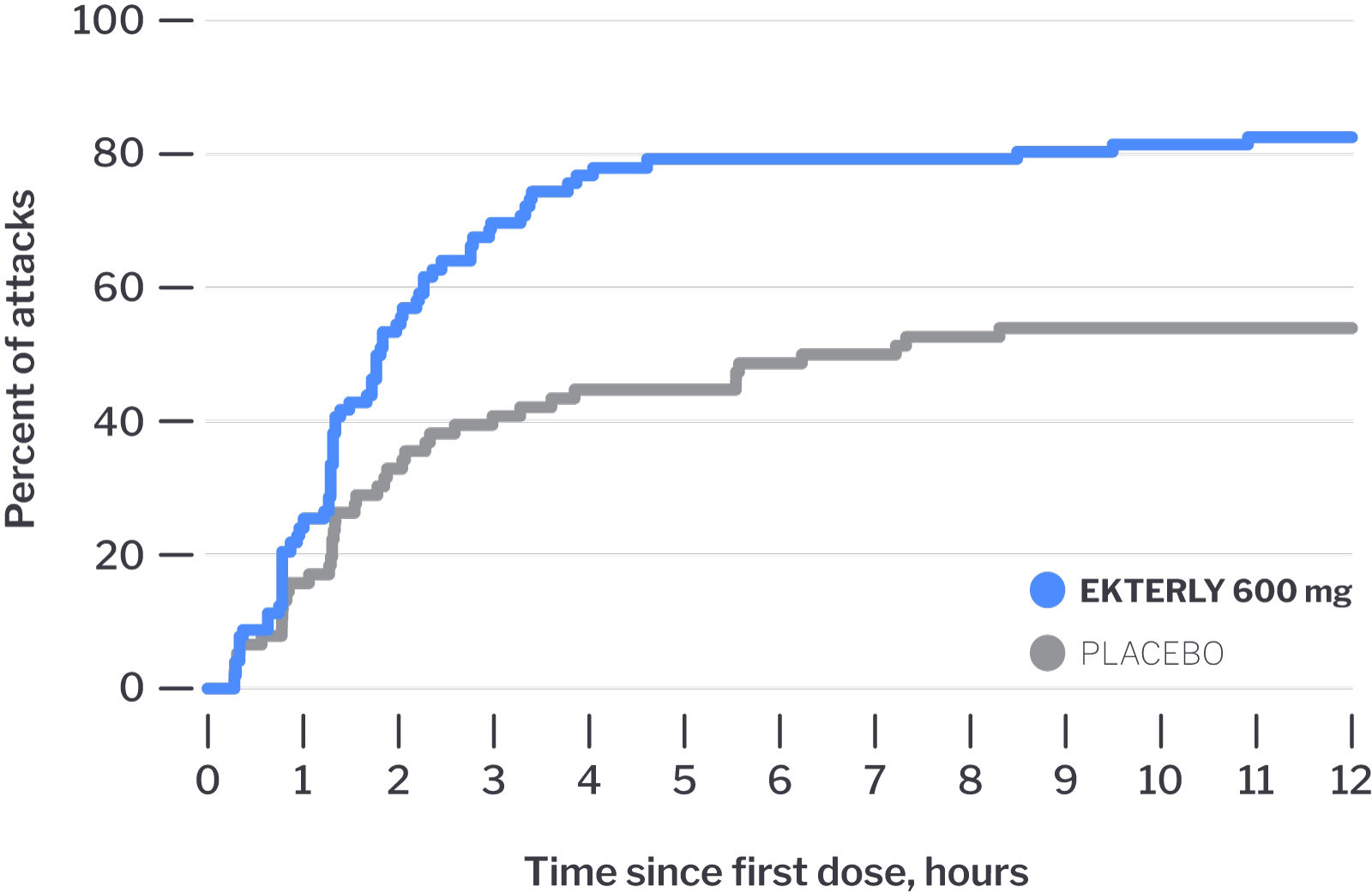
The trial measured how quickly symptoms improved after taking EKTERLY
Median time to the beginning of symptom relief was:
1.8 hours*
WITH EKTERLY
6.7 hours
WITH PLACEBO
*Analysis does not include attacks where response information was not provided. In product label, these attacks were included as having no response to treatment at 12 hours, shifting median to 2.0 hours.
Some people taking EKTERLY began experiencing symptom relief
in
as little as
30 minutes
Time to Beginning of
Symptom Relief
The convenience of 2 pills to treat any attack
The time it took for people to feel relief after taking EKTERLY was similar regardless of:
- Age (12 years and older)
- Whether or not they were on preventative treatment
- Attack location and severity
Works for a wide range of attacks

96% of attacks that
were effectively
treated with EKTERLY required
just 1 dose.


The safety of EKTERLY was similar to placebo
| SIDE EFFECT |
EKTERLY 600 mg n=93 |
PLACEBO n=83 |
|---|---|---|
| Headache, no. (%) | 3 (3.2) | 1 (1.2) |
- No serious side effects related to EKTERLY were reported
- No one dropped out because of side effects
In the KONFIDENT-S trial,
134 patients have treated 1,706 attacks with EKTERLY
At KalVista, our commitment to the HAE community doesn’t end
when
a trial does. That’s why we
created KONFIDENT-S, an
extension of
the KONFIDENT trial. In this new study, all participants
receive
EKTERLY—there’s no placebo—and because it’s open-label, each
person knows exactly what treatment they’re getting.
KONFIDENT-S
gave patients continued access to a treatment they’re
helping
to bring closer to approval, while also allowing us to learn
more about how it works over time. It’s one more way we’re
standing beside this community—every step of the way.
The safety profile in KONFIDENT-S was consistent with that seen in the KONFIDENT trial.
In KONFIDENT-S, laryngeal attacks had a median time to beginning of symptom relief of 1.3 hours. There was no reported difficulty swallowing EKTERLY during laryngeal attacks.


